Why Are Recombinant Proteins Important for Biotechnology and Medicine?
Recombinant proteins play a vital role in biotechnology and medicine. These proteins are engineered through genetic manipulation, offering significant advantages over traditional methods. According to a report by Allied Market Research, the global recombinant protein market was valued at approximately $42.7 billion in 2020 and is expected to reach $64.6 billion by 2027. This growth highlights their increasing importance in various applications.
One major application of recombinant proteins is in therapeutics. Many biopharmaceuticals, such as insulin and growth hormones, are recombinant proteins. The use of recombinant insulin revolutionized diabetes treatment. However, challenges remain, such as ensuring consistent quality and efficacy in production. The potential for allergic reactions and immune responses also requires careful consideration.
In diagnostics, recombinant proteins facilitate the development of sensitive assays. These proteins serve as crucial components in tests for infectious diseases and genetic disorders. Despite their benefits, researchers face hurdles in scaling up production. Addressing these challenges will be essential to fully harness the potential of recombinant proteins in improving health outcomes worldwide.

Importance of Recombinant Proteins in Biotechnology
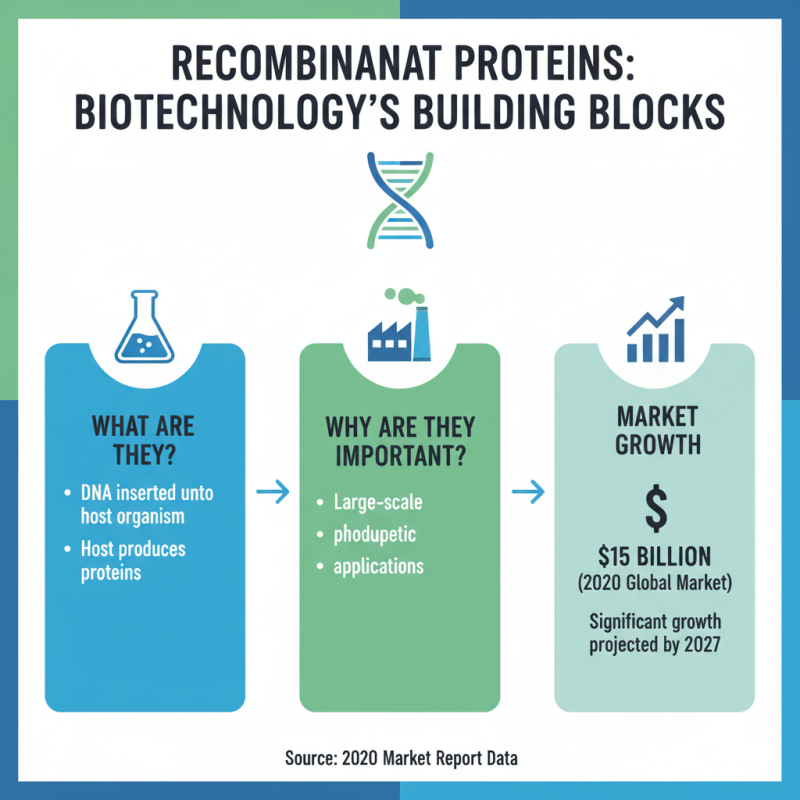
Recombinant proteins play a crucial role in biotechnology. These proteins are produced by inserting DNA into a host organism. This method allows for large-scale production. For example, in 2020, the global recombinant protein market was valued at approximately $15 billion and is expected to grow significantly by 2027. Companies are increasingly relying on these proteins for therapeutic applications.
In medicine, recombinant proteins have transformed treatment options. They are used in vaccines, insulin production, and monoclonal antibodies. The production of recombinant insulin changed diabetes management. However, challenges remain. The high costs of production can limit accessibility. Additionally, issues with protein folding and functionality arise during production. Researchers need to address these challenges to improve overall efficiency.
Not all recombinant proteins are effective. Some may trigger immune responses or fail to bind properly to targets. These shortcomings highlight the need for ongoing research. It’s important to optimize production processes and ensure quality control. Innovating in this field can lead to better therapies and improved patient outcomes.
Applications of Recombinant Proteins in Medicine
Recombinant proteins play a vital role in modern medicine. These proteins are engineered to mimic natural ones, offering targeted treatments. For instance, insulin, a hormone crucial for diabetes management, is produced using recombinant DNA techniques. This method provides consistent quality and supply, unlike traditional extraction methods.
Another significant application is in vaccines. Recombinant proteins are used to create antigens that stimulate the immune system. This approach enhances vaccine safety and efficacy. The hepatitis B vaccine, for example, uses a recombinant protein to trigger an immune response without exposing individuals to the virus. However, this technology has its challenges, including potential immunogenicity concerns.
Therapeutic antibodies are also derived from recombinant proteins. These biologics are tailored to target specific diseases, including cancers and autoimmune disorders. Yet, their development can be expensive and time-consuming. It raises questions about accessibility and affordability for patients.
The journey of recombinant proteins is impressive but not flawless. It requires ongoing reflection and innovation to fulfill its promise in healthcare.
Mechanisms of Recombinant Protein Production
Recombinant protein production is a crucial process in biotechnology and medicine. It involves inserting a gene of interest into an expression system. This can be bacteria, yeast, or mammalian cells. These organisms then produce the desired protein. This method enables large-scale production, making it cost-effective.
The mechanisms of recombinant protein production vary. For instance, bacterial systems are often used due to their rapid growth and simplicity. However, they may struggle with complex proteins. In contrast, yeast and mammalian cells can modify proteins more accurately. This can enhance their activity and stability. Yet, these systems are slower and more expensive.
Challenges remain in optimizing production. There can be issues with protein folding, solubility, and yield. Not all proteins express well in every system. Researchers must often experiment to find the best conditions. These trials can be resource-intensive. However, the potential benefits are significant. Recombinant proteins can lead to breakthroughs in treatment and therapies. They hold great promise for the future of medicine.
Importance of Recombinant Proteins in Biotechnology and Medicine
| Dimension | Description | Example |
|---|---|---|
| Production | Recombinant proteins are produced using DNA technology to insert a gene into host cells. | Insulin production in E. coli |
| Therapeutic Use | These proteins can be used to treat diseases or improve health conditions. | Recombinant monoclonal antibodies for cancer therapy |
| Research Applications | They serve as important tools in molecular biology and genetic research. | Green fluorescent protein (GFP) for visualization in cells |
| Vaccine Development | Recombinant proteins can be used to create safer and more effective vaccines. | Recombinant hepatitis B vaccine |
| Cost-Effectiveness | Production using recombinant techniques can be more economical than traditional methods. | Bulk production of therapeutic proteins |
Challenges in Recombinant Protein Development
Recombinant proteins are critical for advancing biotechnology and medicine. However, developing these proteins is fraught with challenges. The first hurdle is the complexity of protein folding. Incorrectly folded proteins may lose their biological function. This often leads to extensive research to find optimal conditions for proper folding.
Another significant issue involves expression systems. Each system comes with unique advantages and disadvantages. For instance, some systems may not modify proteins correctly. This leads to the need for extensive screening and modifications. Research teams often need to spend months refining their approaches.
Tip: Always document each step in your experimental process. This helps in understanding what works and what doesn’t.
Purification of recombinant proteins is another challenge. Achieving high purity levels can be resource-intensive. Often, scientists must rely on trial and error. This can result in wasted resources and time. Striking a balance between yield and purity remains a common struggle.
Tip: Consider using automated systems for purification. They can save time and reduce human error.
The path to successful recombinant protein development is riddled with obstacles. Despite these challenges, the potential benefits make it a worthwhile endeavor. Researchers must remain adaptable and learn from each trial.
Importance of Recombinant Proteins in Biotechnology and Medicine
This chart illustrates the key challenges in the development of recombinant proteins, including production efficiency, purification difficulties, and stability issues. Understanding these challenges is critical for advancing biotechnology and medical applications.
Future Directions in Recombinant Protein Research
Recombinant proteins hold immense promise for biotechnology and medicine. As research advances, the future of this field looks increasingly exciting. Current estimates show that the global recombinant protein market is projected to reach $52 billion by 2026, driven by innovations and new applications. This growth highlights the need for ongoing research.
Future directions include improving yield and reducing costs. Many production systems, like bacteria and yeast, face efficiency challenges. Current yield rates can be as low as 5-10 mg per liter in some cases. Enhancing these systems would significantly benefit the industry. Additionally, the development of more sophisticated purification techniques could streamline workflows.
Another area worth exploring is the use of artificial intelligence. AI can expedite protein engineering, predicting structures and functions. However, integrating AI into lab practices still presents hurdles. Researchers must navigate the complexities of data reliability and model accuracy. The refining of these methods is crucial for the next wave of breakthroughs. Overall, the path ahead is filled with both potential and challenges.