Top 10 Techniques for Protein Expression and Purification Explained
In the realm of biotechnology, protein expression and purification remain pivotal processes. Dr. Emily Johnson, a leading expert in this field, once stated, "Understanding the nuances of protein expression is essential for successful purification." This highlights the intricate relationship between these two methods.
The journey of protein expression and purification is often riddled with challenges. Researchers encounter various obstacles, from yield inconsistencies to protein stability issues. Each technique, whether it's using E. coli or yeast systems, has its pros and cons. The nuances of optimizing conditions can be overwhelming yet crucial.
Effective protein expression and purification are not merely technical tasks. They demand creativity and problem-solving skills. Scientists must adapt their methods based on the specific protein characteristics. With each experiment, knowledge is gained, yet unexpected outcomes often call for reflection and innovation. This dynamic landscape keeps the field evolving, emphasizing the need for continuous learning and adaptation.
Overview of Protein Expression Techniques
Protein expression techniques are essential in biotechnology and research. They enable scientists to produce proteins in various systems. Commonly used systems include bacteria, yeast, and mammalian cells. Each system has its unique advantages and challenges.
Bacterial expression systems are often favored for their rapid growth and simplicity. However, they may lack post-translational modifications that some proteins require. Yeast offers a middle ground, allowing some modifications but can be more complex to handle. Mammalian systems produce proteins with necessary modifications, yet they require more time and resources.
Progress in protein purification methods is vital. Techniques like affinity chromatography and ion-exchange purification help in isolating proteins effectively. However, achieving high purity can be a tedious task. Sometimes, unexpected contaminants arise, complicating the purification process. Researchers must remain adaptable and mindful of these challenges throughout their work.
Key Factors Influencing Protein Expression Efficiency
Protein expression is a complex process influenced by various factors. Understanding these can significantly enhance expression efficiency. Different host systems, such as bacterial, yeast, or mammalian cells, impact protein yields and quality. For instance, mammalian systems tend to produce correctly folded proteins more efficiently than bacterial systems.
Temperature is another crucial factor. Lowering the temperature during expression can improve protein folding but may reduce overall yield. A study found that tweaking temperature settings led to a 30% increase in soluble protein. This emphasizes the need for careful optimization rather than a one-size-fits-all approach.
**Tip:** Experiment with different expression times. Sometimes, longer expression periods can boost yield. However, over-expression could lead to degradation.
Another vital aspect is the choice of promoter. Strong promoters can enhance expression levels but may also lead to inclusion body formation. It's essential to balance expression strength with proper folding conditions.
**Tip:** Use codon optimization. This can significantly improve translation efficiency in certain host organisms.
Consider the media composition. The right nutrients can enhance growth rates, leading to higher protein yields. One report indicated that media supplements could enhance yields by 50%. Balancing all these factors is challenging but crucial for optimal protein expression.
Common Methods for Protein Purification
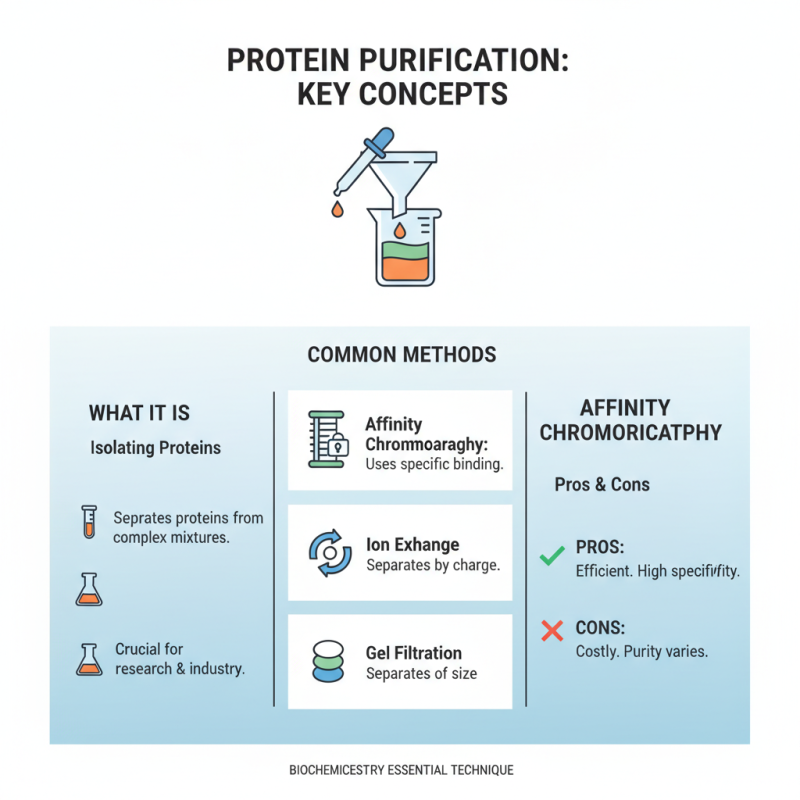
Protein purification is essential in biochemistry. It helps isolate proteins from complex mixtures. Several methods exist, each with its unique advantages and challenges. Affinity chromatography is a popular technique. It uses specific interactions to bind target proteins. This method is efficient but can be costly. Also, it may not always yield pure proteins.
Another common method is ion exchange chromatography. This technique separates proteins based on charge. Adjusting pH and salt concentration can enhance separation. However, it requires careful optimization. A poorly optimized process could lead to unsatisfactory results. Precipitation is also a viable option. It reduces protein concentration but can co-precipitate unwanted substances. Monitoring conditions is crucial to avoid losses.
Size exclusion chromatography rounds out the list of common techniques. It separates proteins based on size. Smaller molecules pass through the beads while larger ones elute first. While generally straightforward, this method may not achieve the desired purity. Balancing speed and purity is a constant challenge in protein purification. Each technique requires reflection and adjustment to improve outcomes.
Comparison of Affinity and Ion Exchange Chromatography
Choosing the right chromatography technique is crucial for protein purification. Affinity chromatography, known for its specificity, uses a ligand to bind the target protein. This method is highly efficient and can yield pure proteins quickly. However, it may not work for all protein types. Sometimes, the binding is too strong, making elution difficult. Adjusting conditions can be tricky and requires careful optimization.
On the other hand, ion exchange chromatography utilizes the charge properties of proteins. It separates proteins based on their net charge at a given pH. This method can be widely applied to various proteins, often allowing for a broader range of applications. However, it may also lead to some protein loss during the process. Factors like pH and ionic strength need constant monitoring, adding complexity to the workflow. Each technique has its strengths and weaknesses, highlighting the importance of choosing wisely based on specific needs.
Top 10 Techniques for Protein Expression and Purification Explained
| Technique | Principle | Applications | Advantages | Disadvantages |
|---|---|---|---|---|
| Affinity Chromatography | Uses specific interactions between a protein and a ligand. | Purification of tagged proteins, antibody purification. | High specificity, effective for complex mixtures. | Potential for elution contamination, cost of matrices. |
| Ion Exchange Chromatography | Separates proteins based on charge interactions. | Initial purification, protein characterization. | High resolution, scalable for large batches. | Requires buffer exchange, can denature sensitive proteins. |
| Gel Filtration Chromatography | Separates proteins based on size using porous beads. | Size-based separations, buffer exchange. | Gentle, preserves protein structure. | Low resolving power for closely sized proteins. |
| Affinitized Membrane Filtration | Uses membrane with affinity ligands for specific binding. | Concentration and purification of target proteins. | Suitable for large volumes, scalable method. | Membrane fouling, limited capacity. |
| Precipitation | Separates proteins by altering solubility conditions. | Initial concentration steps, clarification. | Simple, quick method with low cost. | Risk of co-precipitating contaminants. |
| HPLC | Uses high pressure to push liquid through a column. | Purification of small quantities, analysis. | High resolution, adaptable separation conditions. | Complex setup, requires skilled operation. |
| Salting Out | Increases ionic strength to decrease protein solubility. | Concentration of proteins from solutions. | Cost-effective and simple process. | Lack of specificity, difficult to scale. |
| Ultrafiltration | Separates proteins based on molecular weight cutoff. | Concentration of proteins, buffer exchange. | Efficient concentration, no chemical reagents needed. | Membrane fouling, limited to certain molecular weights. |
| Biodistribution | Uses biological interactions for specific separation. | Purifying enzymes, antibodies, and other biomolecules. | High specificity, low cost of goods. | Requires optimization and may introduce variability. |
| Reverse Phase Chromatography | Separation based on hydrophobic interactions. | Purification of small peptides and proteins. | High resolution, versatile for various applications. | Requires strict buffer conditions, potential denaturation risks. |
Recent Advances in Protein Expression and Purification Techniques
Recent advances in protein expression and purification techniques have revolutionized biotechnology. Innovative methods have improved yields and efficiency. For instance, wave bioreactors are gaining traction. They enhance cell growth by providing a more stable environment. A recent report states that these systems can increase protein yield by up to 30%.
Additionally, membrane chromatography has emerged as a formidable technique. It allows for faster purification with less buffer consumption. This approach saves both time and resources. A study highlighted that this method reduces processing times by 50%. These efficiencies can significantly impact research timelines and costs.
**Tip:** Don't overlook the importance of optimizing culture conditions. Sometimes minor adjustments can lead to significant yield improvements.
Another exciting development is utilizing cell-free systems. These systems can express proteins without living cells. They often speed up the production process dramatically. However, they may not always replicate the post-translational modifications seen in vivo. It's crucial to weigh the pros and cons of this method.
**Tip:** Regularly review and refine your protocols. Continuous improvement can lead to breakthroughs in protein production.

