EPISKIN
EPISKIN, world leader in tissue engineering, offers Human Reconstructed Tissues to the global scientific community – academic and industry – to support research and development activities in Safety and Efficacy
In Vitro Test Approach

ISO 9001

Regulatory context

We provide our worldwide clients RELIABLE AND RELEVANT IN VITRO TOOLS FOR SAFETY AND EFFICACY EVALUATION.
Based in Lyon, France, we partner with cosmetical, pharmaceutical and chemical industries to develop relevant and reliable safety and efficacy tests. Our models are critical tools to speed up Research and Development phases and to shorten the time to market. Some screening tests are already referenced and validated as alternatives to animal use, and others are in the process of validation.
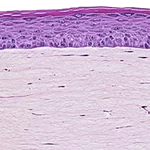
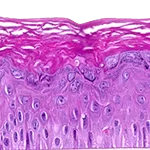
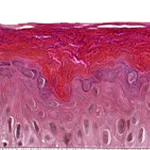
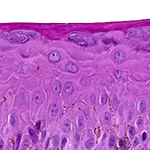
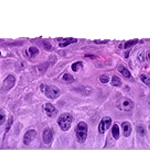
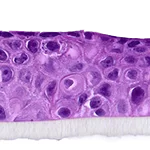
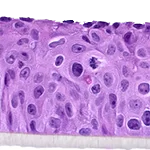
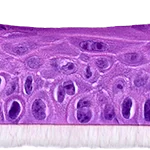
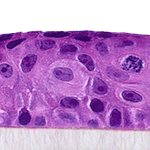
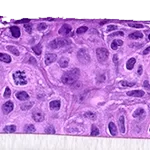
Our unique cell culture process allows to industrialize important volumes of in vitro models of reconstructed human tissues with well characterized histology, functionality and ultrastructure features. The production unit has been designed to deliver every week our clients with thousands of human tissues.
Product Categories
More About EPISKIN
EPISKIN Corporate Movie - ADVANCED 3D MODELS FOR A BETTER, MORE PREDICTIVE AND MORE ETHICAL SCIENCE
Our product lines include:
- Reconstructed human epidermis,
- Reconstructed human epidermis with melanocytes (different complexions are available from phototype II to VI),
- Reconstructed human dermis and epidermis: Full Thickness Model
- Reconstructed Human Corneal Epithelium, Reconstructed Human mucosa: Oral, Gingival, and Vaginal Epithelium.
All of these models allow the evaluation of raw materials, active ingredients and finished products being either in a solid, liquid, powder, cream or gel form.
Flyers and Product Guides

A Comprehensive Array of Multifunctional Cosmetic Claims
Eye Care, Cosmetic & Herbal Soaps, Skin Care, Hair Care & more
Scientific References
FAQ
Q1. What 3D reconstructed human tissue models does Episkin offer?
A. Skin irritation, corrosion, phototoxicity, and ocular irritation models.
Q2. Are Episkin models OECD validated?
A. Yes. Episkin models comply with OECD guidelines and are widely accepted for regulatory testing.
Q3. What are the lead times for ordering Episkin tissue models in India?
A. Standard lead time 4–5 weeks due to production and international shipping constraints.
Q4. How are Episkin products shipped?
A. Temperature-controlled express shipping with detailed handling instructions on arrival.
View More FAQs
Q5. How does EPISKIN ensure the quality and reproducibility of its tissue models?
A. Each EPISKIN tissue batch undergoes stringent quality control for viability, morphology, and barrier function. This ensures consistent, reliable performance and full compliance with OECD and regulatory standards.
Q6. What applications can EPISKIN tissue models be used for?
A. EPISKIN models are used in cosmetics, pharmaceuticals, chemicals, and academic research to evaluate skin irritation, corrosion, phototoxicity, genotoxicity, and compound penetration or efficacy.
Q7. What are the storage and handling requirements for EPISKIN models?
A. Upon arrival, tissues must be stored at 37°C in a CO₂ incubator and used immediately according to the test protocol provided. They are shipped ready-to-use and should not be frozen.
Q8. Do EPISKIN models require ethical clearance for testing?
A. No. EPISKIN models are developed from ethically sourced human keratinocytes and are animal-free, eliminating the need for animal testing or related ethical approvals.
Q9. Can EPISKIN provide customized testing services or model adaptations?
A. Yes. EPISKIN offers customized models and testing solutions upon request, including pigmentation, atopic, and reconstructed ocular tissues tailored for specific study needs.
Didn’t find your answer?
Ask us now.
The Krishgen support team strives to provide swift responses and resolution to your queries. Get in touch for a technical question, product query or quote request.